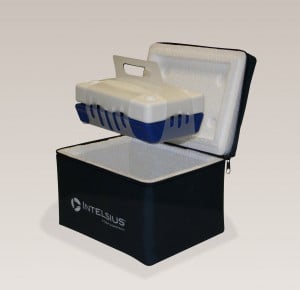
 YORK, England – Intelsius, a DGP company, a designer, manufacturer, and distributor of temperature-controlled packaging solutions, announces an enhancement to its ThermoTrek™ range of solutions. They now feature ThermoCline Technology™ (TCT), allowing 48 plus hours of protection for temperature-sensitive payloads. The TCT addition allows for simple conditioning of a single component, making it easy for patients to use correctly.
YORK, England – Intelsius, a DGP company, a designer, manufacturer, and distributor of temperature-controlled packaging solutions, announces an enhancement to its ThermoTrek™ range of solutions. They now feature ThermoCline Technology™ (TCT), allowing 48 plus hours of protection for temperature-sensitive payloads. The TCT addition allows for simple conditioning of a single component, making it easy for patients to use correctly.
ThermoTrek systems are a range of insulated; patient carry cases designed for the movement of temperature-sensitive medications (2-8 degrees C) either being tested in clinical trials or for approved and marketed medicines used by patients. “Pharmaceutical companies make enormous investments in their IMPs during clinical trials, and the ThermoTrek TCT systems will help patients comply with temperature sensitivity requirements and provide more reliable clinical data outcomes,” said Steve Healy, Intelsius Global Sales Director. In commercially available temperature-sensitive medicines, the system protects the patient’s investment in treatment while ensuring the efficacy of the medicine.
The optional Thermochromic label on the TCT panel visually indicates and verifies the readiness for use. “Keeping patients safe is the primary reason for the ThermoTrek TCT line of products, and simplified conditioning makes it extremely easy for them to use,” said Healy. “In addition, we’ve already had great feedback from leading Pharma companies who are shipping high-value biologics direct to pharmacies here in Europe.”
Every ThermoTrek TCT solution is constructed with high-performance materials which give strong resistance to tears or abrasions while offering stain and water resistance for many months of reliable use. Each case is ergonomically designed to have a minimum impact on the patient’s daily life and enable comfortable medicine transportation. In addition, its discreet design keeps the contents confidential.
About Intelsius
Incorporated in 1998, Intelsius is a subsidiary of DGP Life Science Ltd, headquartered in York, United Kingdom. Intelsius serves many organizations, including government agencies, leading pharmaceutical companies, biopharmaceutical, and clinical research industries to major laboratories and life sciences organizations. With a strong focus on developing environmentally sustainable products and procedures, the company offers solutions to ensure the integrity of customers’ products and samples. Intelsius has a growing global presence with manufacturing facilities, distribution hubs, and local offices worldwide, including North America, Europe, and Asia. For more information, visit www.intelsius.com.
Media Contact: Tammy Moran, Intelsius, 317.536.9950 or tammy.moran@intelsius.com
